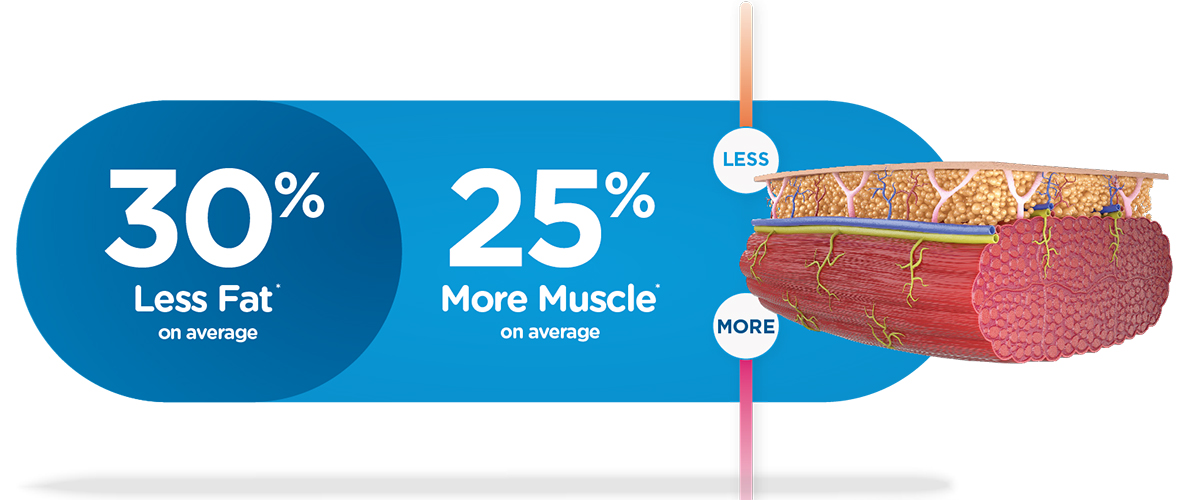
EMSCULPT NEO® – Clinical & FDA-Cleared Applications
For patients who want deeper clinical detail, the following information outlines EMSCULPT NEO’s FDA-cleared indications and how this technology may support rehabilitation, recovery, and functional restoration.
FDA-Cleared Functional Uses
In 2024, EMSCULPT NEO received expanded FDA clearance for stimulating neuromuscular tissue for bulk muscle excitation in the arms and legs for rehabilitative purposes. FDA-cleared indications include:
- Prevention or retardation of disuse atrophy
- Muscle re-education
- Maintaining or increasing range of motion
- Immediate post-surgical stimulation of calf muscles to help prevent venous thrombosis
- Relaxation of muscle spasms
- Increasing local blood circulation
These indications support EMSCULPT NEO’s role in rehabilitation, recovery, and functional restoration.
Clinical Applications
EMSCULPT NEO may be appropriate for post-surgical and rehabilitation patients, individuals recovering from injury or prolonged inactivity, and aging adults experiencing muscle loss. It may also be used when a patient needs muscle re-education and strengthening, or when active individuals are focused on injury prevention and improved performance.
With over 4 million treatments performed worldwide, EMSCULPT NEO continues to expand beyond aesthetics into wellness and functional medicine applications.
If you’re wondering whether EMSCULPT NEO could support your recovery, strength, or movement goals, we’re here to help.
Book a visit at Better Life Chiropractic or schedule a free consultation to discuss your needs and next steps.